Background
The combination of olverembatinib (HQP1351), a novel third-generation tyrosine kinase inhibitors (TKIs), with venetoclax generated high response rates in patients with relapsed/refractory Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph + ALL). However, the efficacy and safety of these two agents-based regimens as frontline treatment remains unknown.
Methods
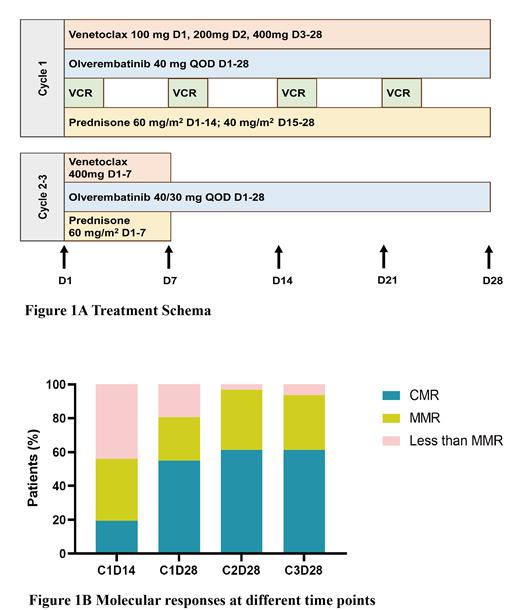
This is a single-arm phase II study (NCT05594784) that enrolled patients (pts) ≥ 14 years (yrs) of age with newly diagnosed Ph + ALL. Pts were required to have an ECOG performance score ≤ 2 and adequate organ function. Pts with accelerated phase or blastic phase of chronic myeloid leukemia were excluded. Pts were treated with a combination of venetoclax (100 mg d1, 200 mg d2, 400 mg d3-28), olverembatinib 40 mg once every continuously other day, vincristine 1.4 mg/m 2 (maximum dose 2 mg) on day 1, 8, 15, 22, and prednisone 60 mg/m 2 on day 1-14; 40 mg/m 2 on day 15-28 in cycle 1. In cycle 2-3, oral treatment with venetoclax 400 mg × 7 days, olverembatinib once every other day continuously and prednisone 60 mg/m 2 × 7 days was administrated in the form of outpatient treatment (Figure 1A). Cycles were repeated every 28 days. Olverembatinib 40 mg once every other day was given during cycle 1 and was reduced to 30 mg once every other day for pts achieving a complete molecular response (CMR). Routine triple intrathecal injection (methotrexate 10 mg, cytarabine 50 mg, and dexamethasone 10 mg) was performed to prevent central nervous system involvement. The selection of subsequent treatment was determined based on the patient's molecular response at 3 months and the availability of donor. The primary end point of this study was the CMR rate at 3 months. CMR was defined as undetectable BCR:ABL1 transcripts by using RT-PCR method with sensitivity of 0.001%. Major molecular response (MMR) was defined as more than 3-log reduction of BCR:ABL1 transcripts.
Results
From August 2022 to April 2023, a total of 31 pts were enrolled. All pts completed 3 cycles of treatment and could be assessed for the primary end point. Th data cutoff date was 25 th July 2023 with a median follow-up time of 5.8 months. The median age was 40 years (range, 20-66 years) and males accounted for 58.1%. Twenty-three pts (74.2%) expressed the p190 transcript and 8 pts (25.8%) expressed the p210 transcript. The median expression level of BCR:ABL1 was 96.33% (range, 70.79%-175.39%). All patients achieved CR at the end of cycle 1 and no tumor lysis syndrome or treatment-related deaths occurred. Molecular response at the end of cycle 1 was CMR in 17 patients (54.8%), MMR in 8 (25.8%), and less than MMR in 6 (19.4%). Molecular response at 3 months was CMR in 19 patients (61.3%), MMR in 10 (32.3%), and less than MMR in 2 (6.5%) (Figure 1B). Of the 10 pts with MMR at 3 months, 8 pts had BCR:ABL1 transcript levels fluctuating between 0.001% and 0.01%. No patients developed relapses or deaths at the last follow-up. The regimen was well-tolerated and safe. Most side effects were grade 1-2. The demand for transfusion and the incidence of infections significantly decreased compared to our historic data treating with intensive chemotherapy plus TKIs. No patient discontinued olverembatinib or venetoclax due to toxicity.
Conclusion
The combination of olverembatinib and venetoclax with reduced-intensity chemotherapy is a safe and effective regimen in patients with newly diagnosed Ph + ALL. The regimen results in high rates of CMR in the absence of intensive chemotherapy or immunotherapy.
OffLabel Disclosure:
No relevant conflicts of interest to declare.
The application of venetoclax in ALL treatment is considered investigational.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal